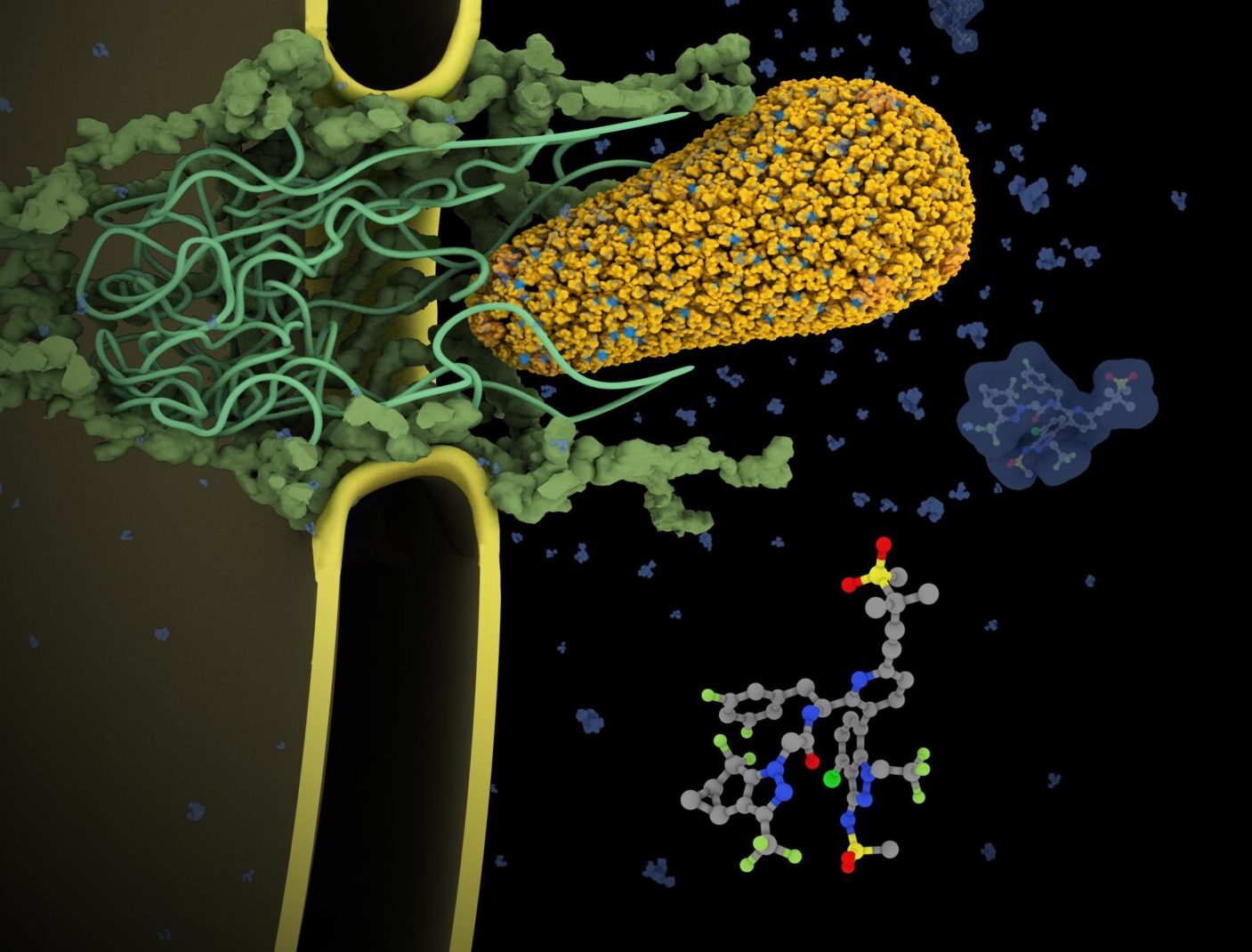
Cracking the Capsid: Lenacapavir and the Next Chapter in HIV Treatment and Prevention
2025 Warren Alpert Foundation Prize Symposium
The Warren Alpert Foundation and Harvard Medical School invite you to our annual scientific symposium, recognizing three scientists whose discoveries led to lenacapavir — a groundbreaking medication that offers new hope for preventing and treating HIV and accelerating the end of the epidemic.


Tomas Cihlar


John Link


Wesley Sundquist
Symposium Program
Each year the recipient(s) of the Warren Alpert Foundation Prize are recognized at a scientific symposium hosted by Harvard Medical School.
Opening Remarks
George Q. Daley, MD, PhD
Dean of the Faculty of Medicine, Harvard University, Caroline Shields Walker Professor of Medicine
Bruce Walker, MD
Director, Ragon Institute of Mass General Brigham, MIT, and Harvard
Award Lectures
Tomas Cihlar, PhD
Senior Vice President of Research, Gilead Sciences
John Link, PhD
Vice President, Medicinal Chemistry, Gilead Sciences (Retd.) Scientific Advisor, Actio Biosciences SAB member, Terremoto Biosciences
Wesley Sundquist, PhD
Professor and Chair of Biochemistry, University of Utah
Invited Speakers
Linda-Gail Bekker, MD, PhD
Professor and Director of the Desmond Tutu HIV Centre, University of Cape Town and Chief Executive Officer of the Desmond Tutu Health Foundation, South Africa
Conversation with Bill Gates
Chair, Gates Foundation, and Founder, Breakthrough Energy and TerraPower, interviewed by Bruce Walker, MD (by video recording)

Sign up to receive updates

For questions about the prize, please contact us.